
Credits: DALL-E
Table of Contents
#009: Can your ALM do this?
Application Lifecycle Management (ALM) is the heart of any software development or validation. I can take it even further. It must be the heart of any validation or qualification.
In my experience which spans over three (3) decades, 90% of the Life Science companies are glued to MS Word or Excel just like ants to honey! All documents whether it is an URS or FDS or a test script is still written in Word, printed and manually executed. We need to ask what an AI chatbot thinks of this fatal attraction!
Some of the life science companies use a standard document management app which is like driving a square peg in a round hole. They are absolutely not designed to manage a Validation or a Software Lifecycle.
Life science companies need a robust, intuitive ALM platform that can manage project of any type (CSV, SDLC, Equipment, Facilities, Process, etc..). The core elements of any ALM (SUT, Requirements, Specifications, Test Cases, Traceability, Risk Management, Coverage Stats, etc..) must be easy to use out of the box and support integration with other apps. This will enable ALM to be the single source of truth.
These are the drivers at xLM to help our customers manage their life cycle in an agile fashion with real time metrics. We have partnered with xQUAL to rollout ContinuousALM.
ContinuousALM: Key Features
Complete Application Life Cycle Management
ContinuousALM lets you manage requirements, specifications, traceability, test cases (both manual and automated) as well as deviations. You can:
Structure your releases down to components
Define your business requirements
Write your functional and technical specifications
Manage your projects using Agile/V-model practices
Design your tests and test case procedures
Orchestrate your test and test plans
Plan and execute your test campaigns
Archive and compare your test results
Report on coverage, results, progress, quality etc.
Track your bugs (integrated or third-party)
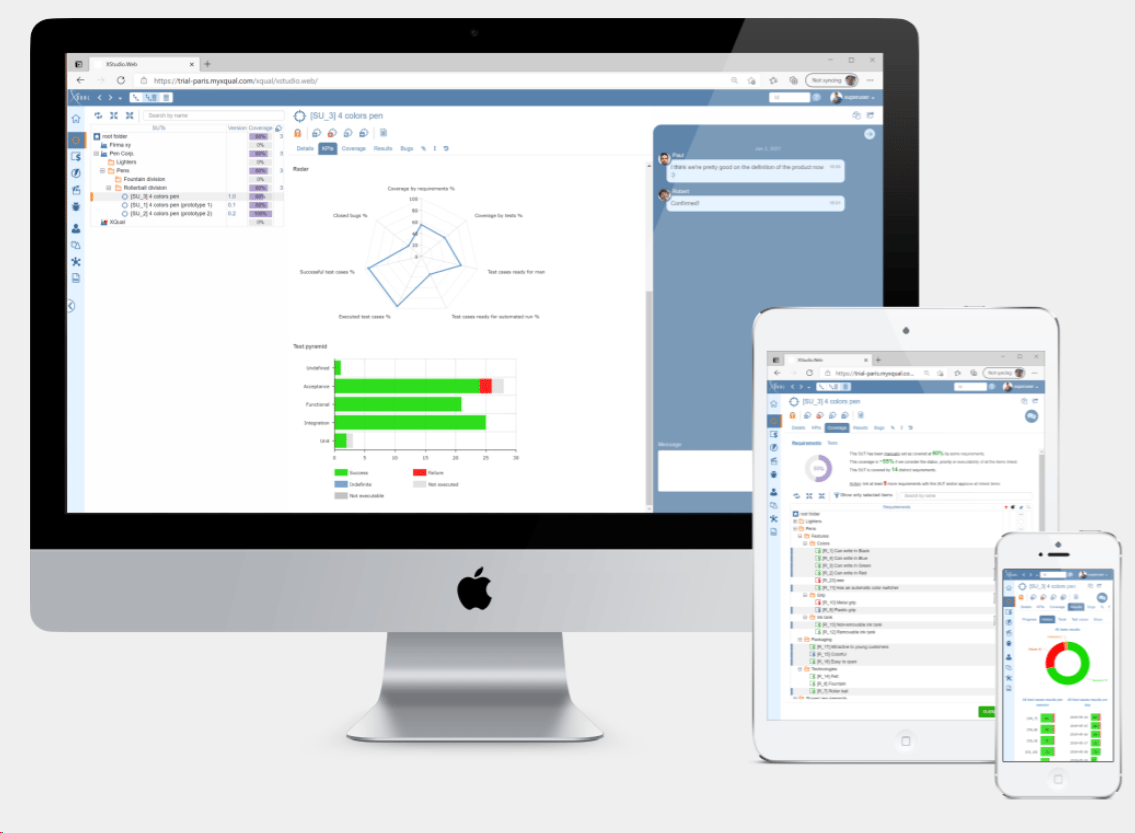
ContinuousALM: A Managed Continuously Validated ALM
System Under Test (SUT) Management
Gain unparalleled insights into your testing activities, ensuring comprehensive coverage and quality metrics. Each SUT, whether it's a hardware device, equipment or a software component, is identified by its name and version, ensuring precision and clarity throughout your testing process.
SUTs are seamlessly organized within a framework of companies, folders, and sub-folders, allowing for systematic organization and management efficiency.
Coverage by Requirements, Specifications, and Tests
With ContinuousALM, you'll have access to comprehensive coverage analysis: Direct coverage by requirements; Indirect coverage by specifications. And let's not forget about indirect coverage by tests, where every aspect of your testing process is factored into to ensure nothing falls through the cracks.
However, what truly sets ContinuousALM apart is the flexibility it offers. Coverage percentages can be manually adjusted based on linked requirements, empowering you to fine-tune coverage levels with precision. And with specification weight determined by priority levels, you can be rest assured that every aspect of your testing process is aligned with your project's goals and priorities.

SUT Coverage by Flow
Requirements and Specifications Management
With our platform, requirements management becomes a breeze, whether you're working directly within Continuous ALM or retrieving requirements from external systems like JIRA, VersionOne, or other third-party systems.
Our requirements tree provides essential information at your fingertips, including the total number of requirements, their distribution across folders, and the status of each requirement—indicated by intuitive icon colors. Moreover, with our coverage column, you'll gain insights into the completeness of requirement coverage, empowering you to make informed decisions. And with customizable coverage indicators accessible via the Coverage drop-down menu, you can tailor your view to focus on specifications or tests, ensuring every aspect of your project is accounted for.
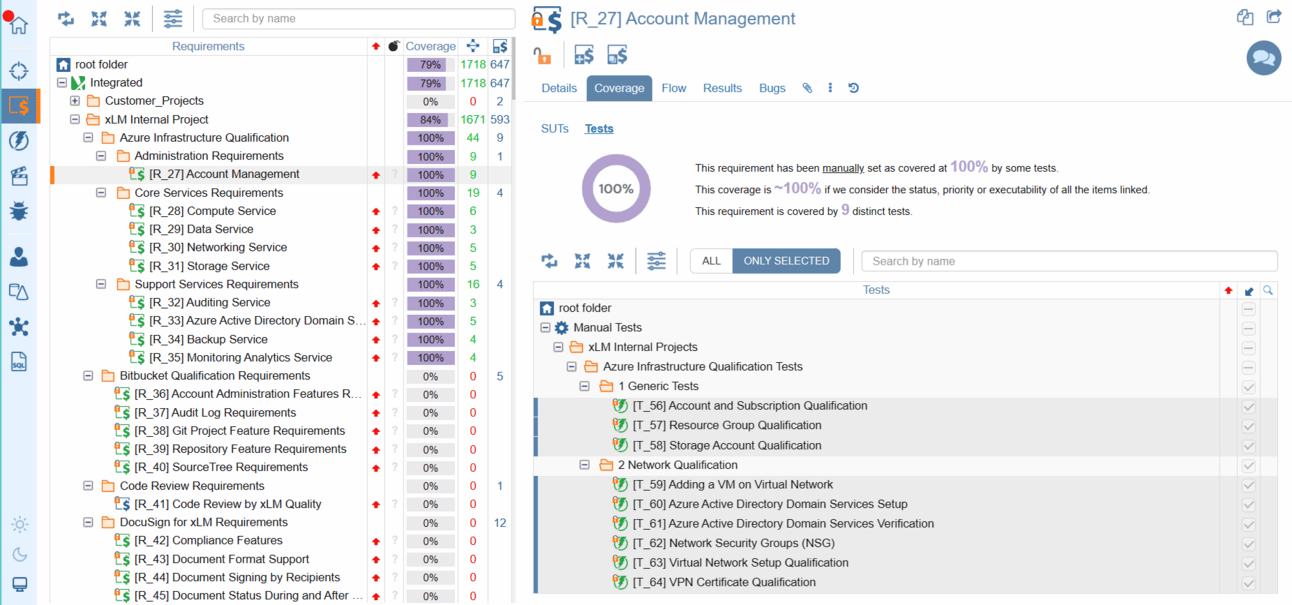
Requirements Coverage Matrix

Requirements Statistics
Requirement Risk Management
You can assign a risk level to each requirement. Not only does it streamline reporting processes, but it helps you in selecting and prioritizing tests. Imagine creating a test campaign and having the system automatically select tests based on specific criteria like risk.
The risk is calculated from the failure probability and the impact of a failure on the functionality. In addition, the probability is calculated from the Frequency at which the feature will be used, the Complexity of its implementation and the Maturity of the its components.
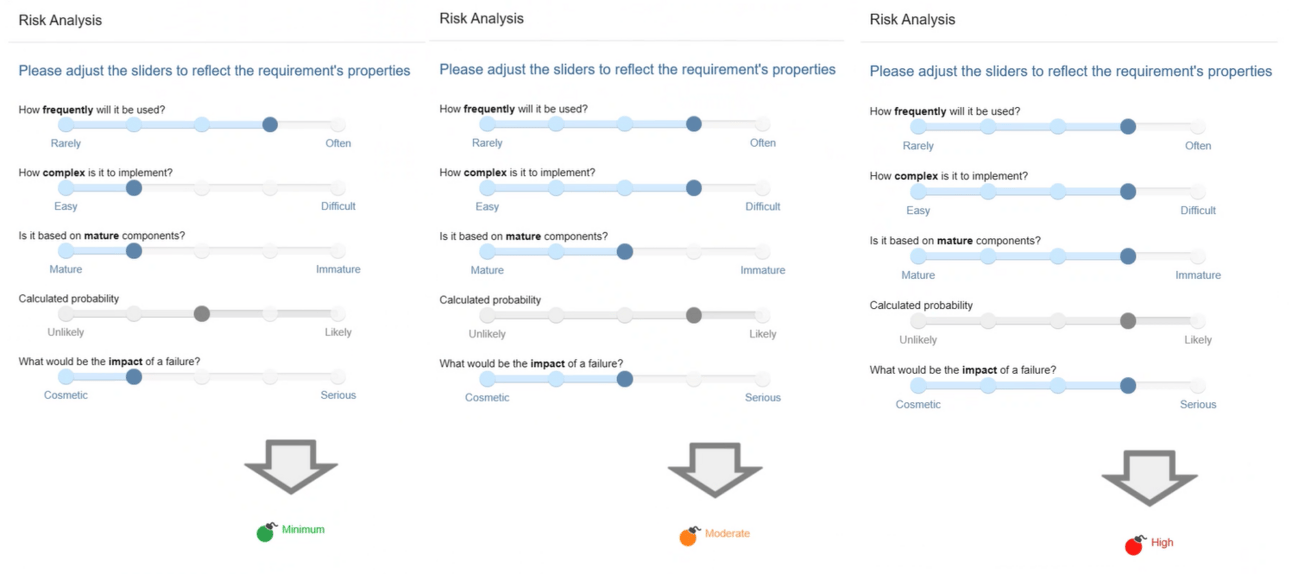
Risk Analysis
Test Management
With ContinuousALM, organization is key to success. Tests are arranged in specific folders following the same structured approach as specifications. However, for software APIs, tests take on a whole new level of precision, with each test meticulously organized per function/method and grouped by function categories. This not only simplifies test suite extension but also paves the way for stress tests, negative tests, and beyond. With ContinuousALM, organization is easy to manage and leverage efficiently.
ContinuousALM's testing module isn't just a tool; it's a powerhouse of features designed to empower teams to ensure comprehensive testing and maintain the highest standards of software quality.
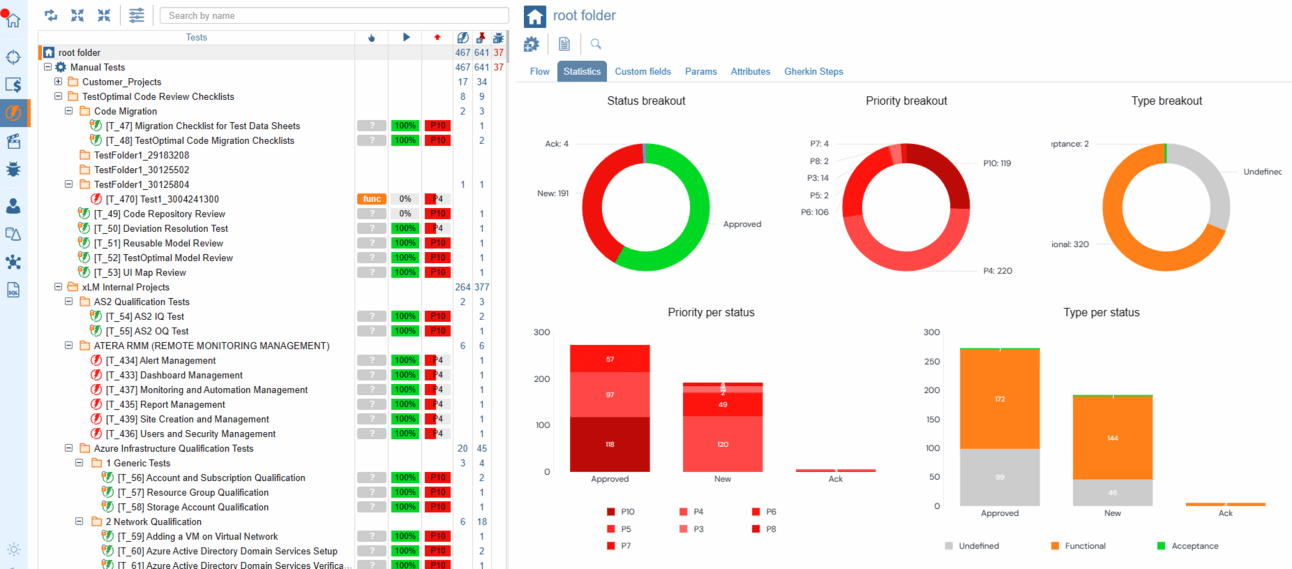
Statistics for Tests and Test Cases
Test Campaign Management
With ContinuousALM's campaign module, organizing, executing, and evaluating test campaigns has never been easier. Our campaigns tree features a coverage column that goes beyond mere estimates, offering insights into the quality of each campaign and session. With quality indicators providing a percentage estimate of test success, you'll have a clear picture of your system under test performance. Moreover, with quality metrics consolidated at the folder/root folder level, you can be rest assured that success is shared across all related tests.
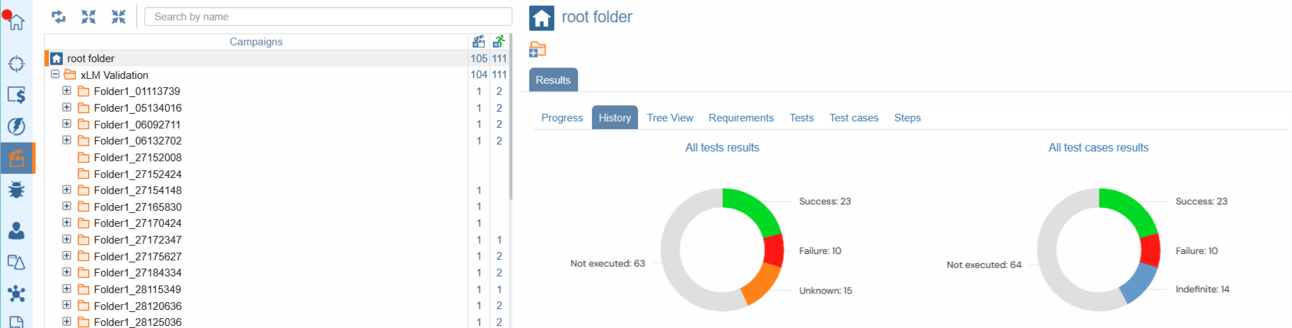
Test Result Campaign Statistics
Defect (Deviation, Discrepancy) Management
With ContinuousALM, every defect finds its home in a centralized repository, ensuring that no bug goes unnoticed. Dive into the defect tree and discover a wealth of essential information, from the total number of defects to their distribution across categories and folders. Moreover, with real-time status updates on each defect—indicated by intuitive icon colors—plus severity and priority ratings for added clarity, you'll have everything you need to tackle bugs head-on.
With ContinuousALM, bug tracking isn't just about identifying issues—it's about taking action. Our platform empowers test operators to conduct thorough report analysis, associating failed test cases with defects to ensure comprehensive tracking and resolution of issues.
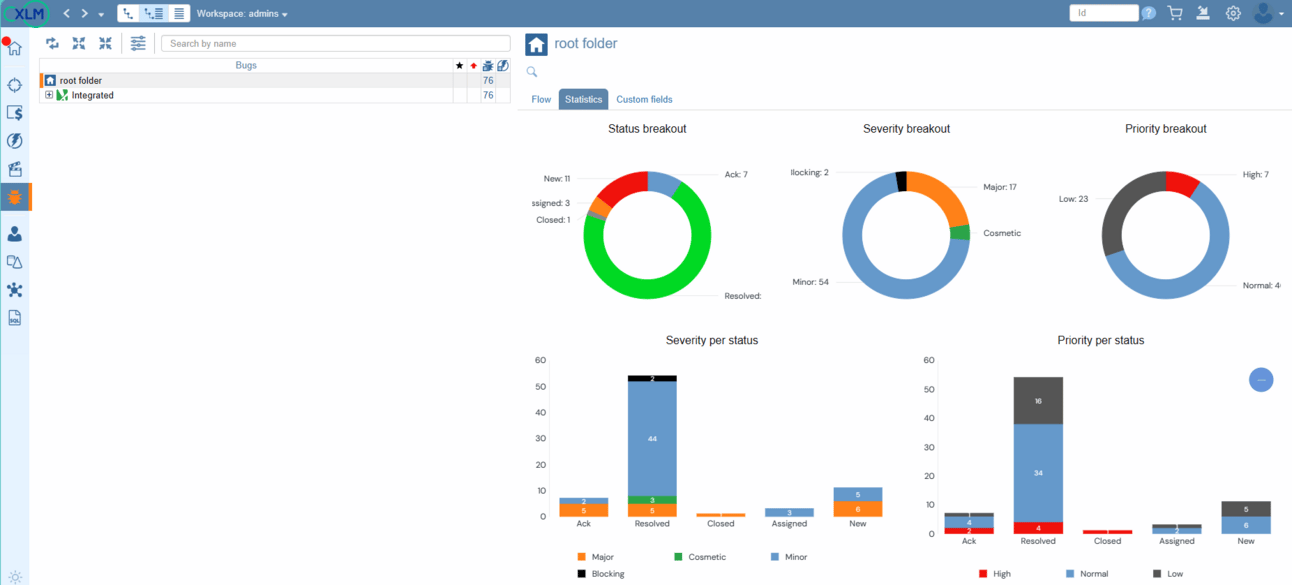
Bug Statistics
Compliance with Part 11
Whether you're exploring the evolution of a System Under Test (SUT), requirements, tests, campaigns, or defects, ContinuousALM puts the entire history at your fingertips.
Part 11 features:
Detailed audit trial at every object level
Granular role based security
Object level version control
Ability for QA to freeze/unfreeze objects
Support for e-signatures
Bi-directional Traceability
Integrated continuous validation at the Product Level and Client Instance Level
Delivered as a continuously validated managed service
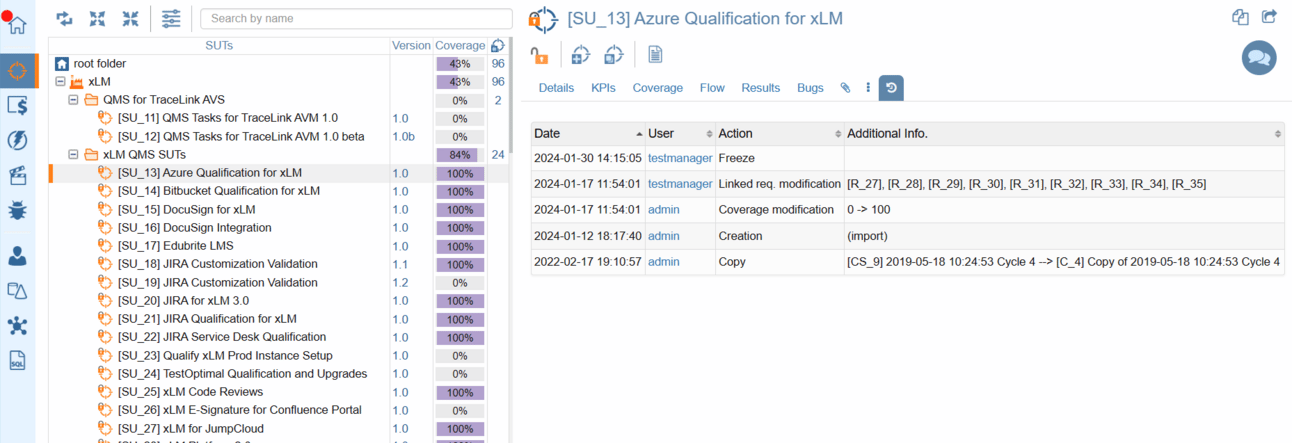
Audit Logs
Traceability, Transformed
ContinuousALM, traceability takes center stage, empowering you to prove the integrity of your lifecycle items with ease. Whether it's ensuring that an item remains unmodified after a certain date or preventing unauthorized changes, our platform has you covered. From SUTs to bugs and everything in between, ContinuousALM's traceability features provide transparency and trust.
Seamlessly trace requirements, specifications, tests, and defects throughout the entire lifecycle. It provides full support for bi-directional traceability
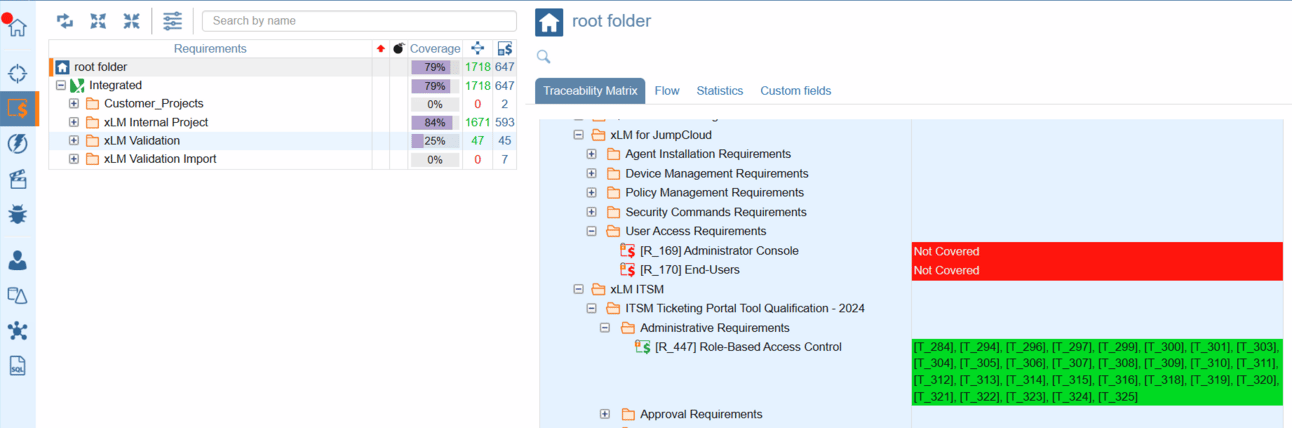
Traceability Matrix
Built-in Support for Part 11 Compliance
From SUTs to tests and beyond, our platform empowers you to freeze items instantly, ensuring their immutability and aligning seamlessly with FDA 21 CFR Part 11 requirements.
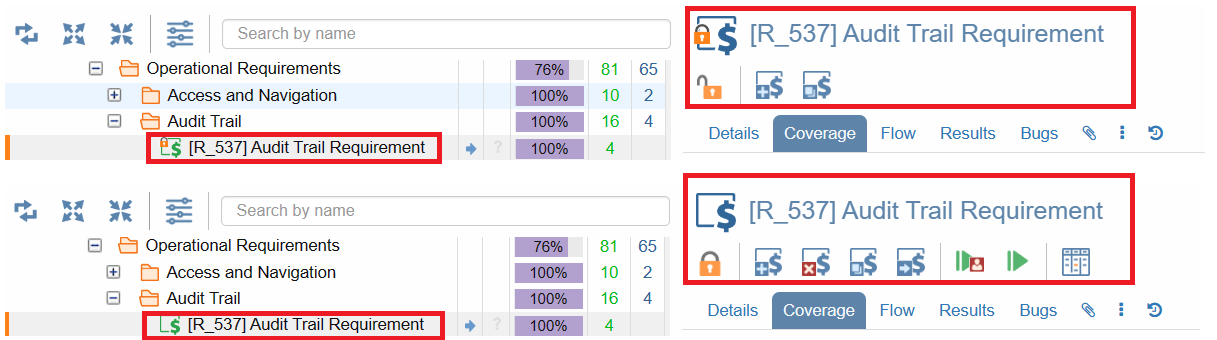
Freezing-Unfreezing
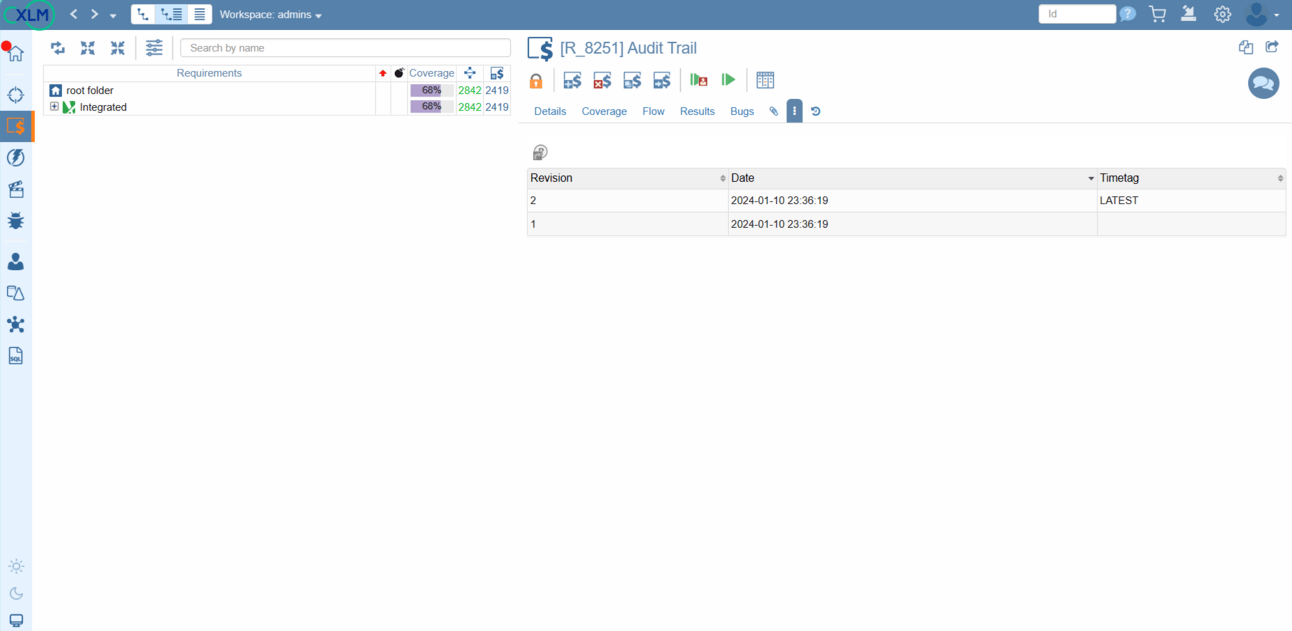
Versioning
Integration Features
Our platform offers flexibility in automation strategies, including an extensive launcher catalog, framework independence, and compatibility with diverse technologies and products. With over 90 launchers at your disposal, executing automated tests has never been easier. Our platform seamlessly integrates with a vast array of automation frameworks and tools, including QTP/UFT, Selenium, JMeter, and many more.
Our tech stack offers a comprehensive set of connectors to integrate with requirements management to bug tracking, automation, and data import capabilities. With dedicated plugins for JIRA, Quality Center (QC), and seamless SQL connections to Mantis, integrating with your favorite tools has never been easier.
Plus, our platform seamlessly integrates with a variety of CI tools, including Bamboo, Jenkins, TeamCity, and more.
Reporting Redefined
From system functionality to test results, compliance status, and risk mitigation strategies, our platform provides comprehensive insights that empower stakeholders to make informed decisions.
Generating User Requirements Specifications (URS), Test Plans, and Traceability Matrices has never been easier. There is built-in support to generate these reports in Word format using your corporate templates.
ContinuousALM provides in real-time the smart progress charts. It covers scope, testability, quality and specifications. You can easily assess your project or product progress.
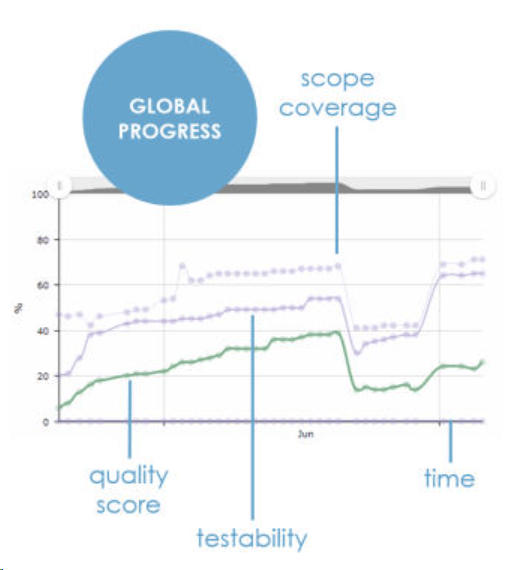
Smart Progress Charts
What's the overall quality of your product, project or system?
ContinuousALM uses complex algorithm taking into account status, priority, executability, local coverage of each component in the traceability matrix, latest results on all the elements to calculate a unique Quality Score
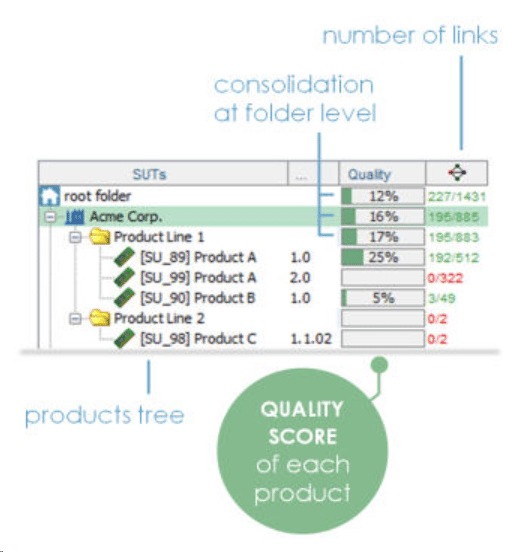
Quality Scoring
Are you improving your quality?
ContinuousALM presents the tests distribution (also called tests pyramid) for each product or project. By visualizing the spread in test types, you can immediately assess if your development cycle supports your quality objectives. You also see how your team is embracing proven practices such as Test-Driven Development (TDD), Behavorial-Driven Development (BDD), etc.
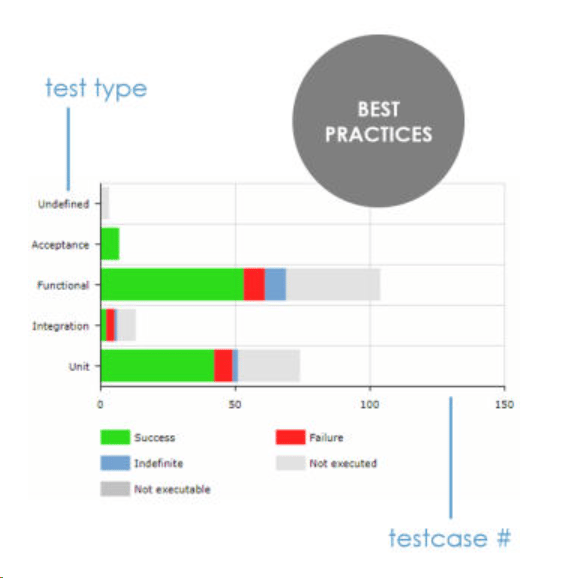
Tests Pyramid
KPIs and Data Visualization
One-click progression assessment on requirements and specifications completion
One-click testability and quality assessment
Radar-based at-a-glance status assessment before system release
Quality-driven assessments using the test pyramid
Dimension-based and time-based data analysis diagrams for any object
Export reports in xml, html, csv (Excel), docx (Word) and pdf formats
System, Requirements, Specification coverage/results
History of all test executions
Campaign coverage/results/trends/progress
Campaign results comparison (per configuration/operator/agent)
Defect (Deviation) metrics per status/per user
Defect submission rate vs resolution rate trend
Graph of dependencies between tests
Tracking of test readiness for manual/automated execution
Tracking of bugs status (submission rate vs resolution rate)
Tracking of scrum projects (velocity graph automatically generated)
Generation of coverage/results metrics on any sub-domains of requirements, specifications and tests
Automatic generation of the traceability matrix
Software Quality Built-in
Support for automated and manual tests execution
Dedicated exploratory testing module based on Session-Based Test Management (supports FDA CSA)
Test case parameterization (including an implementation of the pairwise algorithm)
Real-time execution graph stored
Dependencies between tests
Automatic scaffolding of tests from Requirements or specifications
Support for Bidirectional Traceability including traceability to Campaign Sessions (Execution Runs)
ContinuousALM - Delivered as a Managed Service
In every service we offer, the software app is continuously qualified. Also the customer's instance is continuously validated. In each run, 100% regression is performed.

Continuous Validation Features
AI Features (Coming Soon)
An AI agent will automatically generate test cases from exploratory tests.
An AI algorithm will validate the traceability to ensure accuracy and coverage.
An AI agent will determine the tests to be run based on the changes.
and more....
Conclusion
If you are looking at managing your software, validation or qualification life cycles, then ContinuousALM delivers a rich feature set that can get you going out of the box. Since it is delivered as a Managed Service, you can be up and running the same day. You don't have to worry about any validation document or executing any test protocol. It is delivered as an app that is continously validated.
When you take the rich feature set, real-time KPIs, bi-directional traceability, useful reports, built-in Part 11 compliance and its ability to integrate with any enterprise app, there is only one option for a robust ALM: ContinuousALM.
Latest AI News
What questions do you have about artificial intelligence in Life sciences? No question is too big or too small.
ContinuousALM is built-on xQUAL. xLM, LLC incorportes best practices, automation, reporting tools and configuration settings to make it GxP / 21 Part 11 compliant. xLM LLC delivers ContinuousALM as a continuously validated app to its customers.
ContinuousALM FAQs
Question | Answer |
|---|---|
1. What is ContinuousALM and what are its key features? | ContinuousALM is a comprehensive Application Lifecycle Management (ALM) platform designed specifically for the life sciences industry. It moves beyond traditional document-centric approaches, providing a centralized hub for managing all aspects of software development and validation. Key features include: Complete Application Life Cycle Management: Manage requirements, specifications, test cases (manual and automated), traceability, deviations, and more in one platform. System Under Test (SUT) Management: Organize and manage SUTs, whether they are hardware, equipment, or software components. Coverage by Requirements, Specifications, and Tests: Gain insights into coverage levels with adjustable percentages and priority-based specification weighting. Requirements and Specifications Management: Easily manage requirements within the platform or integrate with external systems like JIRA and VersionOne. Requirement Risk Management: Assign risk levels to requirements, streamlining reporting and prioritizing tests based on risk assessment. Test Management: Organize tests efficiently with structured folders, API-specific organization, and support for various test types. Test Campaign Management: Effortlessly organize, execute, and evaluate test campaigns with insights into campaign quality and session success. Defect (Deviation, Discrepancy) Management: Centralized defect repository with real-time status updates, severity and priority ratings, and integration with test case analysis. Compliance with Part 11: Comprehensive support for 21 CFR Part 11 requirements, including audit trails, role-based security, version control, e-signatures, and more. Traceability, Transformed: Seamless bi-directional traceability between requirements, specifications, tests, and defects, ensuring comprehensive documentation and transparency. Built-in Support for Part 11 Compliance: Features like object freezing, versioning, and audit trails ensure compliance with regulatory requirements. Integration Features: Connect with a wide range of tools, including JIRA, Quality Center, Mantis, and various CI tools like Bamboo and Jenkins. Reporting Redefined: Generate comprehensive reports on system functionality, test results, compliance status, risk mitigation, and more. Software Quality Built-in: Support for automated and manual testing, exploratory testing, test case parameterization, real-time execution tracking, and more. ContinuousALM - Delivered as a Managed Service: Continuously qualified software and continuously validated customer instances ensure reliability and compliance. |
2. How does ContinuousALM address the limitations of traditional document-centric approaches in the life sciences? | Traditional reliance on MS Word and Excel for managing validation and software lifecycles presents several challenges: Lack of Centralization: Documents scattered across various locations lead to version control issues and difficulty in tracking progress. Limited Traceability: Connecting requirements, specifications, tests, and defects across disparate documents is cumbersome and prone to errors. Manual Processes: Heavily reliant on manual documentation, review, and execution, which increases the risk of human error and delays. Compliance Challenges: Ensuring Part 11 compliance with manual document-based systems is time-consuming and requires extensive documentation. ContinuousALM addresses these limitations by: Providing a centralized platform: All lifecycle artifacts are managed within the system, eliminating scattered documents and improving version control. Enabling seamless traceability: Bi-directional traceability features connect all elements, providing a clear audit trail and ensuring compliance. Automating processes: Automated test execution, report generation, and other features streamline workflows and reduce manual effort. Built-in Part 11 compliance: Features like audit trails, e-signatures, and object freezing simplify compliance and reduce the risk of regulatory issues. |
3. What is SUT Management in ContinuousALM and why is it important? | SUT Management in ContinuousALM allows you to effectively organize and manage all your Systems Under Test (SUTs). Each SUT, whether it's a hardware device, a piece of equipment, or a software component, is clearly identified by its name and version. This is crucial because it: Ensures Clarity: Clear identification prevents confusion and errors during testing. Provides Structure: SUTs are organized within a hierarchy of companies, folders, and sub-folders, making them easy to locate and manage. Facilitates Comprehensive Testing: A well-defined SUT inventory ensures all components are accounted for and thoroughly tested. |
4. How does ContinuousALM handle Risk Management? | ContinuousALM features a dedicated risk management module that allows users to assign a risk level to each requirement. This risk level is calculated based on the probability of failure and the potential impact of that failure on the functionality. Risk management is essential because it: Prioritizes Testing Efforts: By focusing on high-risk requirements, teams can ensure that critical areas are thoroughly tested. Improves Resource Allocation: Resources can be directed towards mitigating high-risk areas, optimizing efficiency and effectiveness. Enhances Decision-Making: Risk assessments provide valuable information for making informed decisions about development and validation activities. |
5. What are the benefits of using ContinuousALM for Test Management? | ContinuousALM's Test Management module provides several advantages: Structured Organization: Tests are organized in folders, making it easy to manage and locate specific tests. API-Specific Organization: For software APIs, tests are organized per function/method, facilitating test suite extension and specialized testing. Comprehensive Test Coverage: Support for various test types ensures thorough testing, including functional, stress, and negative testing. Automated Test Execution: Integrates with various automation frameworks, streamlining test execution and reducing manual effort. Real-Time Test Tracking: Track test execution progress in real time, providing immediate insights into test results. |
6. How does ContinuousALM ensure compliance with 21 CFR Part 11? | ContinuousALM is designed with built-in features to meet the stringent requirements of FDA 21 CFR Part 11. Key compliance features include: Audit Trails: Detailed audit logs track all changes made to the system, ensuring accountability and transparency. Role-Based Security: Granular access controls restrict actions based on user roles, preventing unauthorized modifications. Version Control: All objects are version controlled, allowing for easy tracking of changes and rollbacks to previous versions. E-Signatures: Electronic signatures provide secure and verifiable approval processes, ensuring data integrity. Object Freezing: Critical objects can be frozen to prevent further modifications, ensuring data integrity and compliance. |
7. How does ContinuousALM’s reporting functionality benefit life sciences companies? | ContinuousALM offers advanced reporting capabilities that provide valuable insights for decision-making and compliance: Comprehensive Reports: Generate detailed reports on various aspects of the lifecycle, including system functionality, test results, compliance status, and risk assessments. Customizable Templates: Reports can be generated using customizable templates to align with company branding and specific reporting requirements. Real-Time Data Visualization: Visual dashboards provide real-time insights into project progress, test coverage, and quality metrics. Export Options: Reports can be exported in various formats (XML, HTML, CSV, Word, PDF) for easy sharing and integration with other systems. Compliance Documentation: Effortlessly generate documentation required for regulatory submissions, such as URS, Test Plans, and Traceability Matrices. |
8. What future innovations are planned for ContinuousALM, particularly in the area of Artificial Intelligence (AI)? | ContinuousALM is committed to ongoing innovation, and AI is playing an increasingly significant role. Planned AI features include: Automated Test Case Generation: AI will generate test cases automatically from exploratory testing sessions, saving time and improving test coverage. Intelligent Traceability Validation: AI algorithms will validate traceability, ensuring accuracy and completeness of links between requirements, specifications, tests, and defects. AI-Driven Test Selection: An AI agent will determine the most relevant tests to run based on code changes, optimizing testing efficiency and reducing redundant efforts. These AI features are designed to further enhance ContinuousALM's capabilities, automate manual processes, and empower life sciences companies to achieve higher levels of efficiency, quality, and compliance. |
9. How can I get started with ContinuousALM? | If you're ready to experience a streamlined, efficient, and compliant application lifecycle management solution, contact xLM to learn more about ContinuousALM and how it can benefit your organization. |


