
Table of Contents
These programs offer unique opportunities to accelerate development, improve quality, and reduce time-to-market, whether you're developing new treatments or scaling production for commercialization. Your application must be data-driven, technically sound, and future-ready to fully leverage these benefits.
This is where xLM’s AI/ML-powered solutions come into play—helping you meet and exceed FDA expectations with robust, compliant, and intelligent systems.
1.0. FDA programs designed for innovation
The FDA offers several forward-looking programs to accelerate the adoption of innovative manufacturing technologies in the pharmaceutical and biologics sectors. These initiatives—Advanced Manufacturing Technologies (AMT) Program, Emerging Technology Program (ETP), Platform Technology Designation (PTD) Program, and Advanced Technologies Team (ATT) Program—support early engagement, streamlined regulatory pathways, and faster commercialization. AI and machine learning technologies, particularly those enabling real-time validation, predictive analytics, and scalable digital platforms, are well-positioned to support your entry to these programs.

2.0. xLM’s AI-Driven Digital Factory for FDA-Aligned Manufacturing
With opportunities ahead, you need more than a great idea—you need the right technology partner to navigate validation, compliance, and data integrity. That’s where xLM comes in.
2.1. xLM's AI-driven solutions for FDA-aligned manufacturing
2.1.1. Continuous Intelligent Validation (cIV)
Automates GxP validation using adaptive AI models that respond to software and procedural changes in real time, transforming static, paper-based validation into a dynamic, digital compliance framework.
FDA Program Fit:
Accelerates tech transfer under AMT and ETP
Enables scalable, audit-ready digital validation systems
2.1.2. Predictive Analytics & Maintenance
a. Continuous Predictive Maintenance (cPdM)
AI/ML models analyze equipment data to detect early signs of failure and optimize PM schedules. This proactive maintenance strategy reduces unexpected downtime and lowers CAPEX/OPEX.
FDA Program Fit:
Demonstrates manufacturing resilience for AMT
Enhances operational intelligence under ATT
b. AI-Powered Risk Management & Deviation Analytics
Transforms unstructured data from event logs, batch records, and quality reports into actionable insights. Supports real-time CAPA optimization, reduces human error, and fosters predictive quality systems.
FDA Program Fit:
Strengthens quality systems for Platform Tech Designation and ATT
Aligns with FDA’s focus on digital QMS transformation
2.1.3. Continuous Environmental Monitoring System (cEMS)
Integrates certified sensor technology, real-time alerts, and AI-powered dashboards to monitor temperature, pressure, humidity, and particulate matter across GMP facilities. Ensures compliance with FDA 21 CFR Part 11, EU Annex 11, and GAMP5.
FDA Program Fit:
Ensures data integrity and compliance readiness for audits
Supports QbD and real-time release under all FDA tech programs
2.1.4. Autonomous Manufacturing with Digital Twins
In collaboration with AVEVA, xLM is leading the transition from traditional automation to fully autonomous manufacturing. Utilizing AVEVA’s Dynamic Simulation platform and NVIDIA's Raptor DRL engine, this initiative employs deep reinforcement learning to:
Improve process control and safety
Optimize product quality
Reduce unplanned downtime
Enable data-driven decision-making and predictive maintenance
FDA Program Fit:
These autonomous systems help manufacturers meet FDA's expectations for innovation, Quality by Design (QbD), and supply chain resilience under AMT and ATT initiatives.
2.1.5. The Broader Digital Factory Stack
xLM’s digital factory architecture includes the following modular solutions, all GxP-compliant and aligned with FDA modernization goals:

Together, these platforms provide end-to-end visibility, control, and intelligence, making FDA submissions more robust, auditable, and future-proof.
3.0. Why choose xLM?
Deep GxP expertise: All xLM solutions comply with FDA, EMA, and WHO regulatory standards.
Cloud-native & scalable: Rapid deployment across multiple sites and products.
Validated AI/ML: Transparent, auditable algorithms with continuous learning.
The following table illustrates how xLM’s AI-powered services align with the FDA’s key regulatory programs—AMT, ETP, PTD, and ATT—by enabling real-time validation, predictive analytics, and scalable platform technologies across the pharmaceutical manufacturing lifecycle.
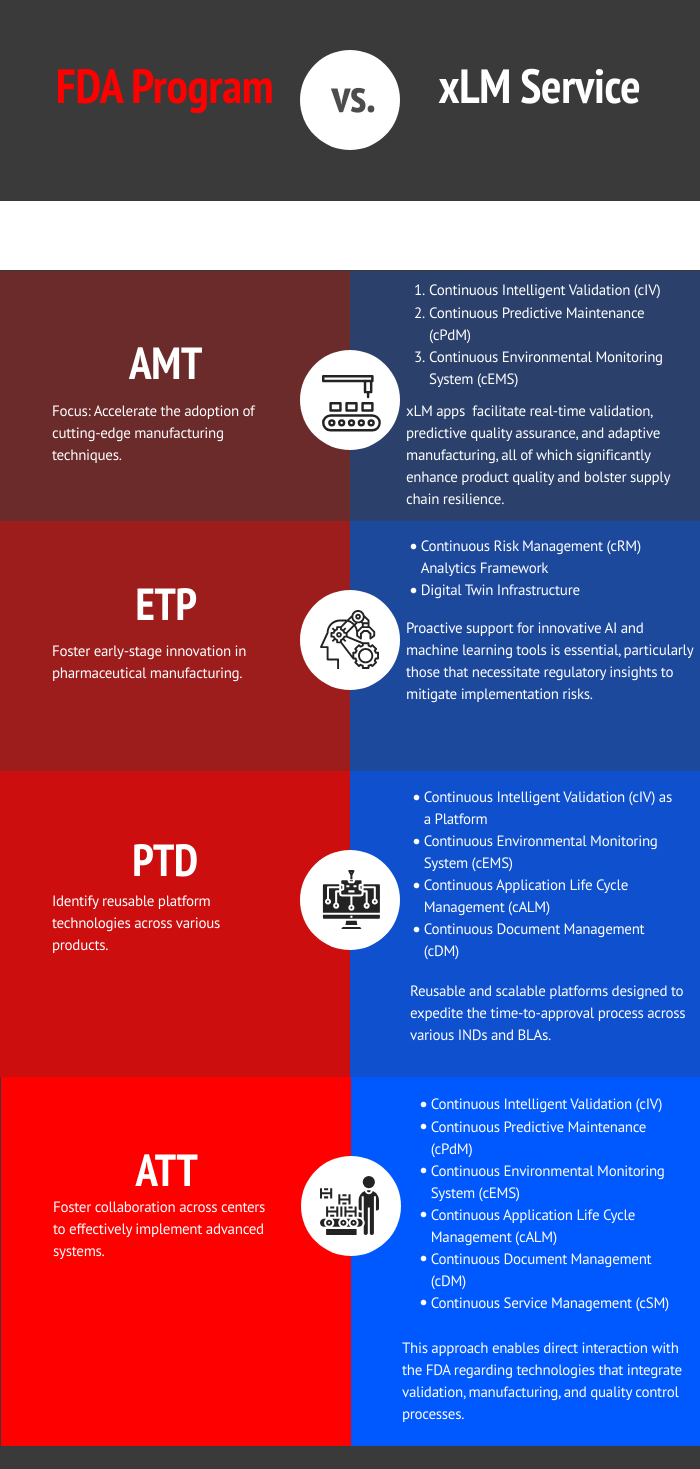
By partnering with xLM, you’re not just adopting technology—you’re integrating regulatory and manufacturing intelligence into your operations.
4.0. Ready to future-proof your FDA submissions?
Whether targeting a single application or building a portfolio, FDA programs like AMT, ETP, Platform Technology, and ATT can be your strategic allies.
Let xLM help you turn these regulatory opportunities into real-world approvals.

