Table of Contents
1.0 The Status Quo: Limitations of Traditional Software Validation
In life sciences — software validation is essential. It guarantees that systems are reliable, safe, and compliant with regulatory standards such as FDA 21 CFR Part 11, EU Annex 11, and GAMP 5.
However, traditional validation methods are characterized by several drawbacks:
Time-consuming: Validation can take weeks to months for each system.
Manual-heavy: Involves cross-functional teams to draft, review, and maintain documents including URS (User Requirements Specification), test protocols, traceability matrices, and validation reports.
Error-prone: Manual processes often lead to compliance risks due to rework and overlooked connections.
Non-scalable: Each new system requires significant effort with limited reusability.

With the evolving software lifecycle and the rise of Agile/DevOps methodologies, this manual approach has become a critical bottleneck.
2.0 Why Continuous Intelligent Validation (cIV) Represents the Future
cIV by xLM signifies a transformative shift in validation philosophy. Rather than viewing validation as a manual step following development, cIV integrates intelligence and automation throughout the entire validation lifecycle.
This is an AI-powered platform—not merely a tool—that autonomously conducts software validation tasks using intelligent agents. From documentation creation to test execution, cIV reduces human effort by over 95% while ensuring comprehensive regulatory compliance.
2.1 Key Differentiators:
Human-in-the-Loop: Quality assurance oversight is included for all approvals.
Autonomous Agents generate, link, and execute validation tasks with minimal input.
Compliance-Ready: Designed to comply with 21 CFR Part 11, Annex 11, and CSA.
3.0 cIV Architecture: Engineered for Speed, Scale, and Compliance
cIV is structured as a modular, microservices-based platform, comprising three core components. Each component is delivered as a managed application and orchestrated through xLM’s cSM (Service Desk) interface.
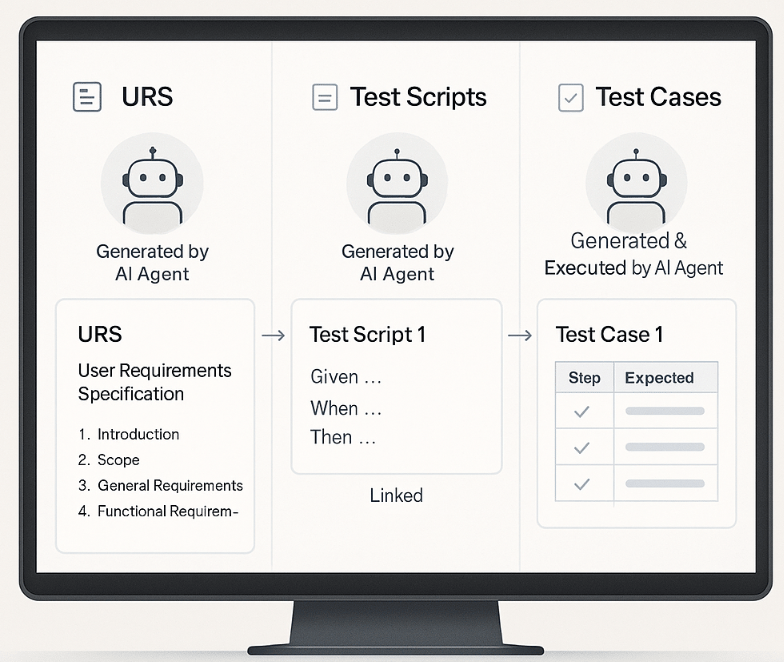
3.1. URS Generation Agent
Accepts source inputs such as user manuals, SOPs, and URLs.
Utilizes LLMs + Retrieval-Augmented Generation (RAG) to draft structured, GxP-compliant URS.
Features an integrated QA layer for review.
Outputs are traceable, version-controlled, and aligned with your corporate templates.
3.2. Test Case Generation Agent
To create automatic test cases, the agent utilizes output from the xTelliGent recorder while performing a dry run that adheres to the custom instructions provided.
This approach ensures that all necessary scenarios are captured accurately, facilitating a comprehensive testing process.
3.3. Test Execution Agent
Consumes test scripts and executes them like a human accessing the browser, desktop client and/or mobile client.
Logs results, evidence, and exceptions into a tamper-proof audit trail.
Generates validation Test Protocol Reports (TPEs) in real-time.
“The result: an intelligent, self-coordinated validation pipeline.”
4.0 Key Features of cIV — Detailed Overview
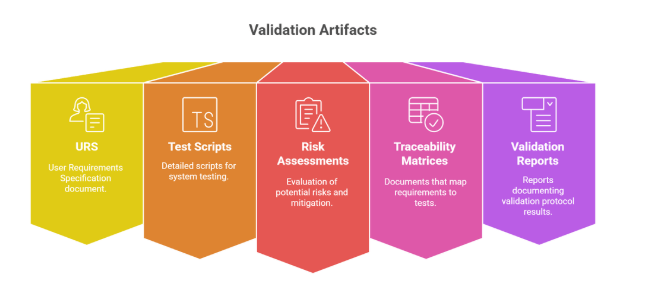
4.1 Autonomous Document Generation
Automatically produces validation artifacts such as:
URS (User Requirements Specification)
Test Scripts
Risk Assessments
Traceability Matrices
Validation Protocol Reports (TPEs)
Outputs adhere to GAMP 5 and GxP Predicate/21 CFR Part/Annex 11.
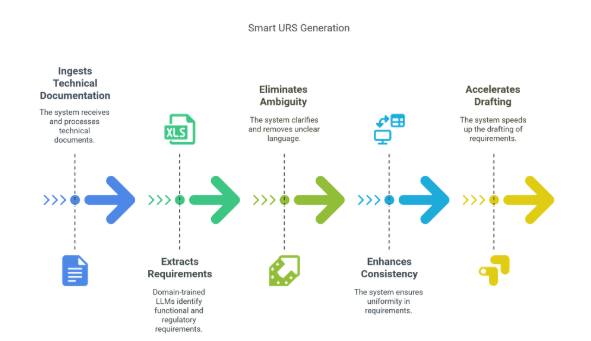
4.2 Smart URS Generator
Ingests technical documentation - multi-modal inputs - audio recordings, PDFs, URLs, meeting transcripts and more.
Extracts functional and regulatory requirements using domain-trained LLMs.
Eliminates ambiguity, enhances consistency, and accelerates requirement drafting.
4.3 Dynamic Risk Calculator
Assigns and monitors risk across requirements and test cases.
Adjusts dynamically based on changes in requirements or execution outcomes.

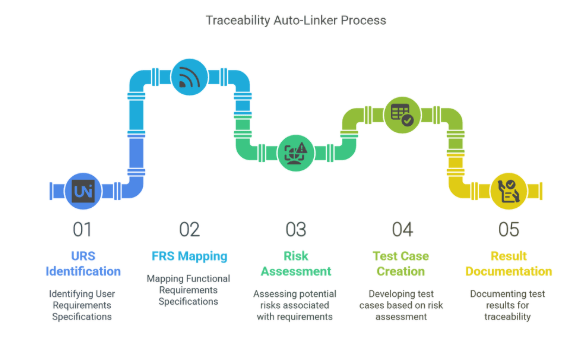
4.4 Traceability Auto-Linker
Automatically maps and links validation elements:
URS → FRS → Risk → Test Case → Result
Ensures comprehensive traceability, crucial for audits.
4.6 Real-Time Audit Trail
Records every action and decision.
Provides zero-effort audit readiness for 21 CFR Part 11 and Annex 11.
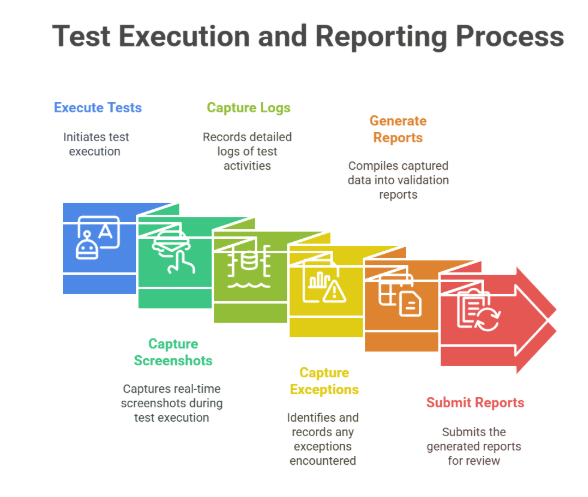
4.7 Execution Engine with Evidence Capture
Executes tests via Agentic AI automation.
Captures real-time screenshots, logs, and exceptions.
Generates validation reports ready for submission.
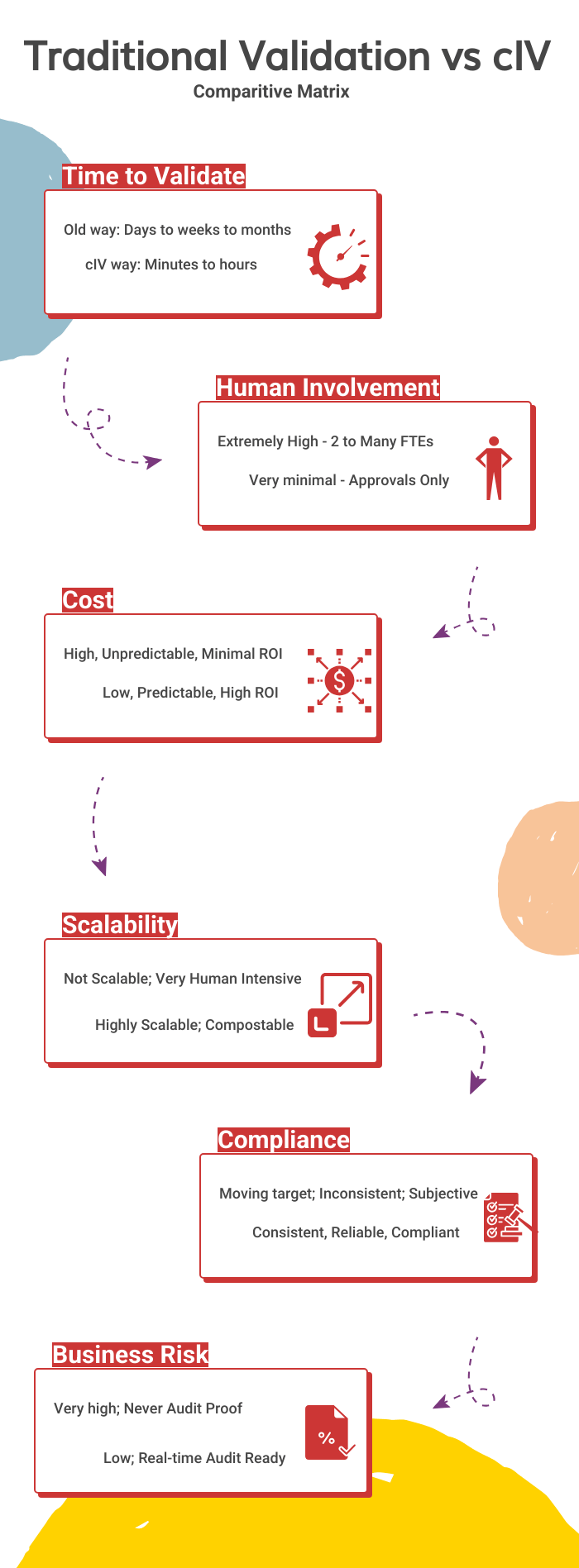
5.0 Traditional Validation vs cIV: A Comparative Matrix
6.0 Final Thoughts: cIV Transforms Validation into a Seamless Process
cIV is not merely automation—it embodies intelligent, autonomous validation.
By integrating advanced AI agents, GxP domain expertise, and regulatory compliance, cIV offers:
Speed: Reducing timelines from months to minutes.
Efficiency: Achieving over 95% automation.
Compliance: Built-in adherence to 21 CFR Part 11 and Annex 11 traceability.
Scalability: Capable of validating across various systems, processes, and environments.
Whether you're facing challenges with time-to-market, overwhelmed by documentation, or gearing up for your next FDA audit—cIV is the future-proof solution for continuous validation.

