
Table of Contents
1. Introduction — 2025 changes the game
The latest State of AI Report 2025 confirms that 2025 is no longer about pilots, experiments, and PowerPoint strategies.
The State of AI Report is the most widely read and trusted analysis of key developments in AI. Published annually since 2018, the open-access report aims to spark informed conversation about the state of AI and what it means for the future. Produced by AI investor Nathan Benaich and Air Street Capital.
It focuses on real production adoption, real revenue, and real competitive separation.
Three insights stand out:
AI consolidates around companies deploying at industrial scale, backed by billions in private investment and vast compute capacity.
Safety and governance lag behind, with external safety budgets far smaller than what labs and enterprises spend to commercialize.
Agentic AI will reshape workflows, commerce, and business models, and industries that move slowly will not catch up.
If 2024 was about learning, 2025 emphasizes execution, scale, and responsible deployment at speed.
This is where AAA — Audit, Automate, Accelerate becomes the essential system layer for pharmaceutical and biotech companies.
2. Pharma Is Now Under Pressure to Scale but Safely
Pharma faces unique challenges today.
Data fragments across multiple systems hinder access to a consistent, reliable single source of truth.
Complex workflows with many steps, dependencies, and manual interventions slow productivity and decision-making.
Compliance is mandatory; every task, decision, and output must meet GxP standards and withstand regulatory inspection.
Regulatory pressure grows as agencies demand stronger controls, continuous oversight, and proof of responsible AI use.
AI outcomes must be explainable, validated, and defensible so regulators, auditors, and stakeholders understand decision processes.
Meanwhile, some competitors advance faster.
They redesign workflows to remove unnecessary steps and simplify processes.
They deploy AI in daily operations rather than limiting it to pilot experiments.
They reduce cycle time by eliminating bottlenecks and automating repetitive tasks.
They improve First-Time-Right performance with AI-assisted decisions that reduce errors and rework.They maintain GxP readiness by embedding controls, documentation, and traceability into every automated step.
These organizations will not just operate better; they will inhabit a fundamentally different competitive reality.
Most companies today possess the key ingredients for AI.
They have models that generate insights and automate decisions.
They have tools to integrate AI into workflows and systems.
They have dashboards that display performance and metrics.
However, they often lack the validated execution layer to expand AI safely, efficiently, and repeatedly across the enterprise.
This gap is precisely what AAA was designed to close.
3. AAA — The Execution Layer for 2025
AAA is a 5-day transformation sprint that moves an organization from AI intention to validated execution.
The structure is simple:
3.1. Audit — Understand the Work, Not Just the Policy
Before transforming processes, the effort begins with a thorough examination of the actual workflow.
Focus on how work runs, not how SOPs or flowcharts describe it.
Analyze handoffs to identify where responsibility transfers cause delays, confusion, or information loss.Measure waiting times to reveal where tasks idle due to pending decisions, missing inputs, or resource constraints.
Review documentation steps to understand how compliance requirements slow operational decisions.
Identify areas where human effort compensates for missing systems, tools, or automation.
Map workflow risks to ensure future AI automation remains safe, controlled, and audit-ready.
Audit is not an AI-readiness checklist; it goes beyond maturity scoring or capability assessment.
It is not a high-level maturity rating, as such scores fail to uncover operational bottlenecks or compliance vulnerabilities.
It is not a generic consulting report; the output is actionable, execution-ready, and tailored to real operations.
Audit maps the real operating truth with timestamps, effort profiles, delays, validation needs, roles, and compliance conditions.

xLM uses cAIC (Continuous AI Consultant) - A platform that can conduct 100s of interviews simultaneously on a 24x7 basis. This enables xLM to gather tribal knowledge not just from a handful of key employees but from all the employees.
Not just gather information, validate in real-time, ask the right questions and analyze information from 100s of sessions to convert it into knowledge.
This creates the baseline for transformation.
3.2. Automate — Deploy AI Where It Is Safe, Valuable, and Measurable
Once workflows are mapped, automation decisions rely on evidence, not enthusiasm.
Decisions rest on validation requirements, ensuring every automated step can be proven and justified to regulators.
Role responsibility determines who owns the decision, approval, and output of each automated activity.
Risk level evaluates potential impact on patient safety, product quality, and regulatory compliance.
Business impact confirms automation delivers measurable operational value.
Cycle time contribution identifies tasks that slow the process and are prime candidates for automation.
Data readiness ensures inputs are accurate, available, and suitable for reliable AI operation.
Governance implications ensure automation fits within documented controls, SOPs, and quality expectations.
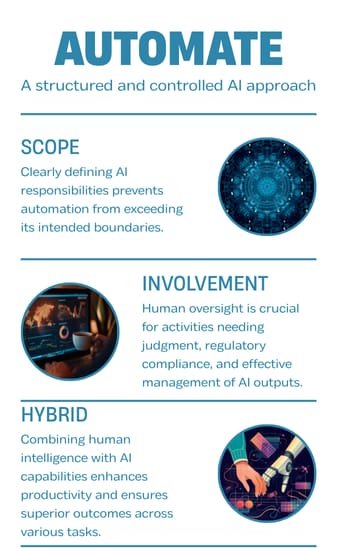
In the Automate phase, the approach remains structured and controlled.
The scope of AI responsibility is clearly defined to prevent automation from exceeding intended boundaries.
Human involvement is specified for activities requiring judgment, oversight, or regulatory responsibility.
Hybrid models leverage human and AI collaboration to deliver superior results.
Required controls surround each automated task, including audit logs, approvals, alerts, traceability, and performance monitoring.
Safety and compliance are not added later they are built into the automation blueprint itself.
3.3. Accelerate — Create an Operating Model That Scales
Accelerate consolidates all elements needed to embed AI as a sustainable organizational capability.
Establish a repeatable deployment model so new AI use cases follow a proven approach.
Define standard operating frameworks to guide development, validation, deployment, and ongoing use consistently.
Apply risk-based controls to ensure automated processes remain compliant and safe, based on workflow criticality.
Create a structured governance model clarifying ownership, accountability, approvals, and decision rights throughout the lifecycle.
Document process definitions so every AI activity is clear, traceable, and aligned with operational procedures.
Implement continuous monitoring to detect performance drift, model failure, or process deviations early.
Establish a measurement framework to track improvements in quality, efficiency, compliance, cost, and operational impact.
Accelerate integrates AI into normal operations, moving beyond isolated proofs of concept.
Adoption no longer requires rebuilding governance and documentation for each new use case.
Compliance remains continuous as automation expands, supported by traceability and documented controls.
Standardized deployment enables teams to move faster without sacrificing reliability or regulatory readiness.
Each new use case launches faster and cheaper because the foundation, controls, and operating system are established.
This is how companies advance from scattered wins to enterprise-level AI scale.

4. Why AAA helps pharma stay ahead in the 2025 AI economy
The report reveals a clear competitive pattern for 2025.
Winners will deploy AI directly into production workflows, not just experimental environments.
They will do so repeatedly, building a system for continuous AI rollout.
They will ensure every deployment remains compliant, traceable, and aligned with regulations.
They will act faster than competitors, reducing time-to-value and accelerating improvements.
They will govern without slowing operations, maintaining oversight without burden.
Pharma does not need more models, pilots, or dashboards that display data without changing outcomes.
The industry needs workflow redesign to make processes streamlined, efficient, and automation-ready.
It needs industrialized deployment, making AI a standardized, scalable operational capability rather than isolated projects.
It needs guardrails that enable speed, providing protection without blocking innovation.
It needs a framework that integrates AI, risk management, and quality into a single operating system.
AAA provides a blueprint for transformation from audit to production.
It delivers controls to maintain compliance, traceability, and responsible automation at every stage.
It provides an operating system that standardizes AI development, deployment, validation, and monitoring.
It offers the speed to move from strategy to results without rebuilding processes each time.
It ensures the defensibility regulators require when automation integrates into GMP, GCP, and quality-critical workflows.
Pharma keeps pace with the AI economy not by copying Big Tech, but by implementing a transformation system suited for regulated manufacturing.

5. Why AAA matters for AI safety and compliance
The report issues a clear warning.
AI capability is increasing rapidly, with more powerful systems arriving at faster intervals.
Investment is scaling at the same pace, pushing organizations to adopt AI aggressively.
However, external oversight and governance are not expanding fast enough to match these developments.
For pharma, this imbalance creates unacceptable exposure in regulated environments.
AAA closes this gap by embedding safety in daily operations.
Safety becomes visible because every decision, step, and output is transparent and trackable.
Safety becomes structured through defined controls, procedures, and responsibilities.
Safety becomes operational, implemented in day-to-day workflows, not left as high-level policy.
Safety becomes audit-ready with complete records, evidence, and traceability for regulatory review.
Safety becomes continuously measurable, allowing ongoing monitoring of performance, quality, and risk.
With AAA, every AI recommendation includes explicit validation logic explaining how the result was produced.
Every use case includes documented control gates defining approval, responsibility, and escalation paths.
Every deployment has a governance wrapper with policies, procedures, and defined operating boundaries.
Every model includes operational traceability so actions, outcomes, and inputs can be reconstructed anytime.
Every automation can be defended to regulators with evidence, documentation, and end-to-end audit trails.
This approach lets pharma move fast, deploy real AI into workflows, and maintain high safety and compliance.

6. Conclusion — the moment to scale has arrived
2025 is no longer about experimentation.
The market is moving.
Capital is flowing.
AI workloads are entering production.
Agent-based automation will change how work gets done.
Organizations that hesitate will spend 2027 catching up.
AAA gives pharma:
A validated path
A structured operating model
A fast execution framework
A system to deploy AI safely, at scale
The AI economy is here.
Now is the time to build the deployment muscle that will define the next decade of competitive advantage.

