1. Introduction — From Periodic Checks to Real-Time Confidence
In 2024-25, the life sciences industry reaches a pivotal moment. Regulatory expectations demand continuous, traceable, and defensible compliance. Pharmaceuticals and biotech firms can no longer rely on periodic temperature-mapping studies, lengthy log-book cycles, manual data-logger retrievals, offline Excel sheets, and tedious report preparation. Regulators now require real-time environmental control, audit-ready traceability, and predictive risk mitigation.
We developed Continuous Temperature Mapping (cTM) not merely as an environmental monitoring tool but as a continuous compliance engine for regulated manufacturing.
With cTM, organizations shift from intermittent snapshots to continuous assurance, providing QA/RA, facility, and manufacturing teams real-time confidence that temperature controls and environmental compliance never pause.

2. The Traditional Gap — Where Periodic Mapping Fails
Periodic temperature mapping traditionally involves placing data loggers for 7, 14, or 21 days, retrieving them after the study, manually downloading data, conducting Excel-based analysis, and preparing a validation report. This method has several limitations:
Long gaps between studies during which conditions may drift.
Manual, labor-intensive processes prone to human error.
Delayed visibility of issues, often discovered well after they occurred.
Reactive compliance problems identified only after manifestation.
For modern GxP operations, this approach is unacceptable. It causes delays, increases compliance risk, and may lead to product losses.

3. Enter cTM — Continuous, AI-Driven, GxP-Ready Temperature Mapping
cTM redefines environmental compliance. Once deployed, it operates 24/7, in real time, with end-to-end automation and compliance built in.
Key elements that distinguish cTM include:
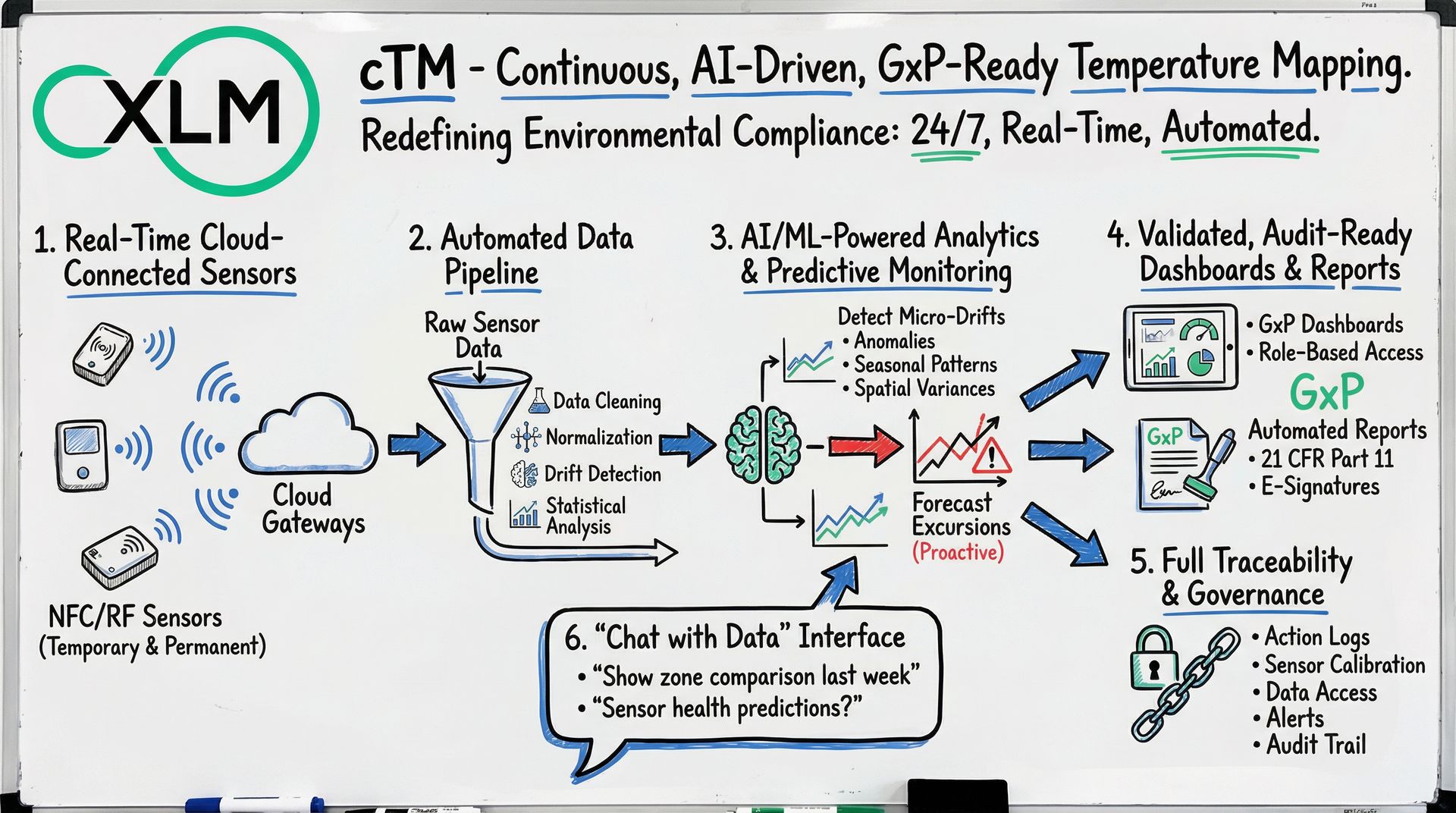
1. Real-Time Cloud-Connected Sensors
Using NFC/RF-based IIoT sensors, cTM supports both temporary (mapping studies) and permanent installations. Sensors auto-connect to cloud gateways, enabling continuous data streaming without manual steps.
2. Automated Data Pipeline
Raw sensor data flows into an AI-powered backend that performs data cleaning, normalization, drift detection, and statistical analysis instantly and automatically. This eliminates manual logger retrievals and offline Excel processing.
3. AI / ML–Powered Analytics & Predictive Monitoring
cTM extends beyond recording temperature. It uses AI/ML pipelines to detect micro-drifts, anomalies, seasonal patterns, spatial variances across zones, and forecasts potential excursions before they occur. This shifts monitoring from reactive to predictive, enabling proactive interventions.
4. Validated, Audit-Ready Dashboards & Reports
Dashboards are purpose-built for regulated life-science environments, featuring role-based access, versioning, audit trails, and compliance controls. Report generation (GxP-compliant) is automated, with templates, change logs, and electronic signatures conforming to 21 CFR Part 11 and Annex 11.
5. Full Traceability & Governance
Every action—from sensor deployment to calibration logs, data access, report generation, and alerts—is logged. This ensures full traceability for audits, inspections, or compliance reviews, substantially reducing risk.
6. “Chat with Data” Interface for Instant Insights
An AI-powered conversational interface lets users query temperature history, zone comparisons, sensor health, and predictions without SQL or Excel. This democratizes data access across QA, engineering, and operations teams.
In summary, cTM automates the entire temperature-mapping lifecycle, embedding compliance, traceability, and continuous monitoring by default.
4. The Value — What cTM Delivers for Pharma & Biotech
cTM offers significant operational and compliance benefits by automating temperature mapping end-to-end. With one-time sensor deployment, real-time data streaming, and instant GxP-ready reporting, teams save substantial time and effort. Automation reduces labor costs, minimizes errors, and eliminates repeated mapping cycles. AI-driven drift detection and predictive alerts enhance risk mitigation, while full audit trails and built-in 21 CFR Part 11/Annex 11 compliance ensure continuous inspection readiness. Real-time dashboards improve transparency across QA, facilities, manufacturing, and leadership. The system scales seamlessly from small rooms to multi-site global operations without re-architecture.

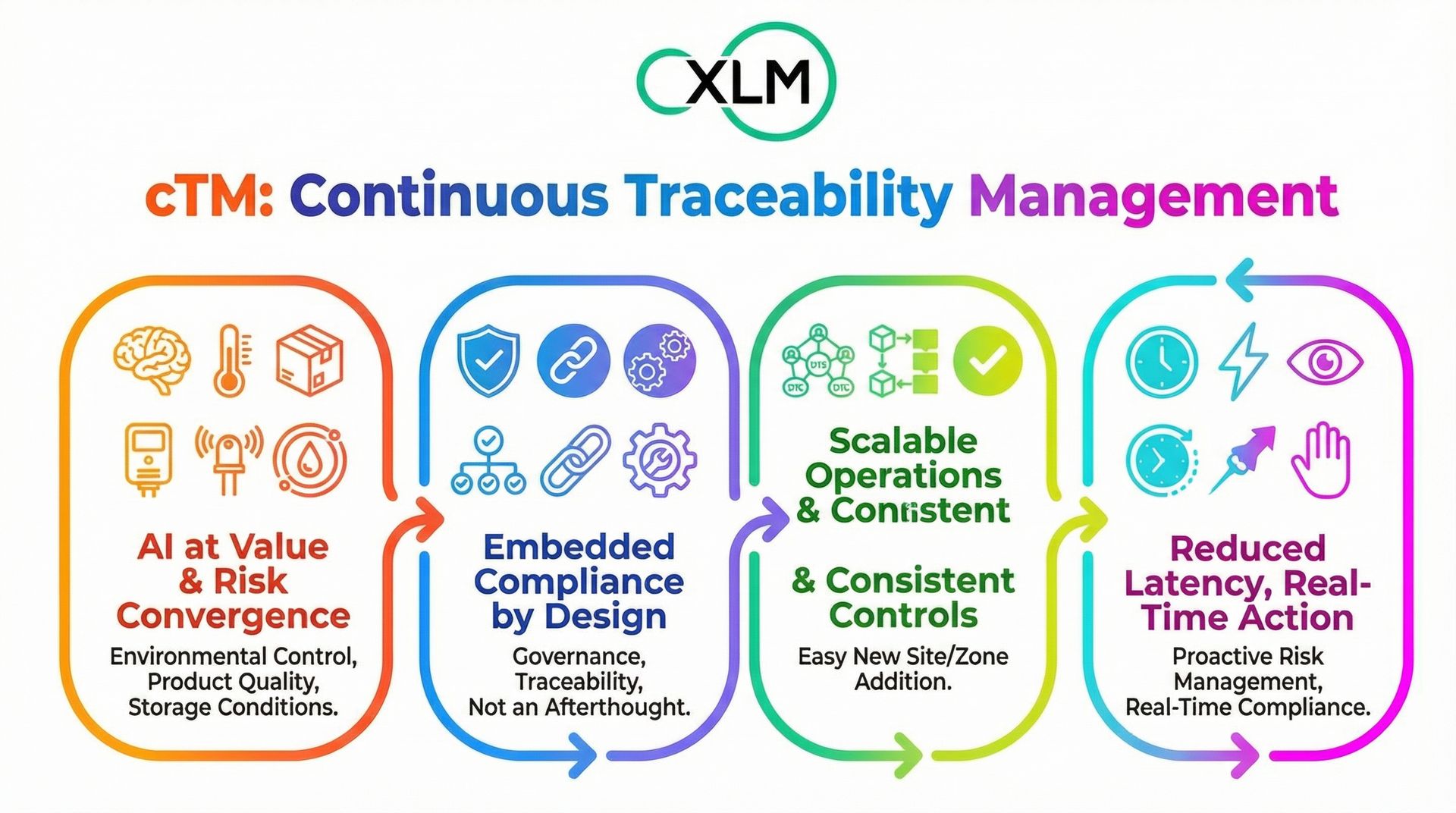
5. Why cTM fits the 2025 AI + GxP paradigm
Industry trends show AI adoption in pharma and biotech is accelerating, but compliance concerns remain a barrier.
cTM aligns with this paradigm:
It deploys AI where value and risk converge: environmental control, product quality, and storage conditions.
It embeds compliance (governance, traceability) by design, not as an afterthought.
It scales across operations with consistent controls; adding a new site or zone is straightforward.
It reduces latency between detection and action, enabling real-time compliance and proactive risk management.
In other words, cTM is not an “AI experiment”. It is industrial-scale automation built for regulated operations, exactly what the 2025 GxP economy demands.
6. Join us — revolutionizing GxP compliance with cTM
To showcase how cTM can transform environmental compliance at your facility and demonstrate live real-time mapping, analytics, and reporting, we invite you to our upcoming event:
Revolutionizing GxP Compliance with Continuous Temperature Mapping (cTM) hosted by xLM.
At the event, you will:
See a live demo of cTM for cold-chain, warehouse, and clean-room environments
Understand how AI-driven analytics and predictive monitoring work in practice
Learn how to deploy cTM quickly and integrate it with your existing QMS and validation workflows
Hear from industry experts about regulatory readiness, audit compliance (21 CFR Part 11 / Annex 11), and best practices
Whether you work in QA, facilities, supply chain, validation, or operations, this session will show how cTM can replace cumbersome periodic studies, reduce compliance risk, and provide continuous assurance.
Don’t miss your chance to transform environmental compliance from a regulatory burden to a competitive advantage.

7. Conclusion — cTM is not a tool. It’s a compliance revolution
As 2025 unfolds, leaders in pharma and biotech will embrace continuous automation, real-time monitoring, and audit-ready compliance as core capabilities, not optional add-ons.
With cTM, xLM offers more than environmental monitoring: we provide continuous assurance, operational transparency, and regulatory readiness.
We invite you to experience this transformation live at our upcoming event and see how cTM can reduce risk, improve efficiency, and future-proof your compliance.
See you at the event.

